- Industries & Machines Industries & Machines
- IIoT IIoT
- Service-Toll Processing Service-Toll Processing
- Material Material
- News News
- IR Information IR Information
-
Sustainability
Sustainability
Sustainability
- Introduction
- Hosokawa Micron Group "Basic Human Rights Policy"
- Hosokawa Micron Group "Basic Policy on the Environment"
- Sustaibality Policy - Mission Statement
- Editorial Policy
- Integrated Report
- Materiality & Strategy
- Technological contribution to a sustainable global environment
- Contributions towards a safer, more secure and prosperous society
- Sophistication of governance that supports business
- ESG Data Collection
- Sustainable Business Management ~ Finance
- Infromation Disclosure Based on TCFD Recommendations
- Jobs and Careers Jobs and Careers
-
About Us
About Us
About Us
- Greetings (Company Introduction)
- Hosokawa Micron Group "Basic Human Rights Policy"
- Hosokawa Micron Group "Basic Policy on the Environment"
- Management Philosophy
- Corporate Overview
- Executive Officers
- Corporate Profile
- Business Areas and Strengths
- Corporate History
- Hosokawa Micron Group
- Domestic Facilities
- Overseas Subsidiaries (Asia)
- Overseas Subsidiaries (Europe)
- Overseas Subsidiaries (America)
- Asian Agents
- Powder Technology Research Institute
- Annual Publication "Micromeritics"
- Industrial Property Rights
- Journals and Books
- Technical Information
- Compliance Charter
- Privacy Policy
- Cookie Policy
- Quality Principle

Industries & Machines
- TOP
- About Us
- Technical Information
- Details of Millling technology
- How things break
- Greetings (Company Introduction)
- Hosokawa Micron Group "Basic Human Rights Policy"
- Hosokawa Micron Group "Basic Policy on the Environment"
- Management Philosophy
- Corporate Overview
- Executive Officers
- Corporate Profile
- Business Areas and Strengths
- Corporate History
- Hosokawa Micron Group
- Domestic Facilities
- Overseas Subsidiaries (Asia)
- Overseas Subsidiaries (Europe)
- Overseas Subsidiaries (America)
- Asian Agents
- Powder Technology Research Institute
- Annual Publication "Micromeritics"
- Industrial Property Rights
- Journals and Books
-
Technical Information
- Summury of Powder Technology
- Details of Millling technology
- Details of Classification Technology
- Details of Mixing/Blending Technology
- Details of Particle design Technology
- Details of Agglomeration Technology
- Details of Drying Technology
- Details of Dedusting Technology
- Details of Measuring Technology
- Compliance Charter
- Privacy Policy
- Cookie Policy
- Quality Principle
How things break
How things break
Why does it take a certain amount of force to break an object? Why does the amount of force depend on the object to be broken? What does it mean for something to break?" These are fundamental questions, but it would be a long story to explain them. I could write a book about it.
The author of this page is aware of a number of general books, including
"Why Things Break: Understanding the World By the Way It Comes Apart" by Mark E. Eberhardt
etc. may be interesting.
If you are interested in learning more about the subject, we recommend that you read books in the area of materials strength science.
Most of them will start with a discussion of crystal transitions, so have a look around. I don't think there were many equations (at least not in that area). The level of this course is about the same as that of an undergraduate student of science and technology at university (second or third year).
I'll try to explain it with images here.
Solid structure
In general, solids and solid-like materials (e.g. glass) are made up of atoms and molecules that are bonded together to form a shape.
When this solid or solid-like material is subjected to a crushing force, the force does not act uniformly on the whole material or on the bonds between the atoms that make up the material.
Some of the atoms and molecules that make up the material are not "bonded" together in a regular way. It is in these areas that the forces act and cause the breakdown. This is because the bonds in these areas are weaker than in other areas.
To give you an idea of this, consider a jungle gym for example. The points of intersection are evenly spaced and the angle of intersection is a constant 90 degrees (although it is 3D). If one of the intersection points is slightly out of position, that part will look distorted. Doesn't it look weak? Other defects include missing intersections (defects), or a whole block being pushed over like a gap in a fault (dislocations).
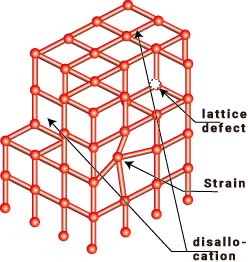
When these intersections are made up of atoms or molecules, we have crystals. In high school you will probably only deal with atoms, but in organic materials such as polymers and medicines, the molecules are bonded together in such an orderly way that they are called crystals. How are they bound together? In technical terms, they are held together by forces known as hydrogen bonds and van der Waals forces. For more information, please refer to a chemistry book. Although not an organic substance, when water forms solid crystals, i.e. ice, the water molecules are strongly bound to each other by hydrogen bonds.
These irregularities are called "dislocations", and the bonding strength of the dislocations is very weak, being less than a few hundredths of the strength of the bonds between atoms or molecules that are regularly bonded.
Fracture, or shattering, can be thought of as the breaking of a weak bond, which distorts the surrounding bonds, which in turn are weakened by the distorted bonds, and so on. In other words, the crack propagates and eventually breaks.
If you have read this far, you may have heard of beating a metal to make it harder. You may be thinking, "Isn't it the other way around, that when a force is applied, it concentrates on the dislocation and causes it to crack? You may think it is the other way around.
First of all, a dislocation is created when you hit it (the atoms move). Dislocations move when a force is applied to them, but dislocations have a tendency to repel each other. As the number of dislocations increases, the dislocations that are free to move are blocked by the other dislocations that have already been created, and they are unable to move towards each other. Now, I mentioned earlier that if the dislocations don't move and the interatomic distance doesn't open up, the material doesn't shatter. This means that the material becomes harder. This method of increasing the number of dislocations deliberately to make the material harder is known from the forging of Japanese swords (the dislocations are not created directly by hammering, but by allowing carbon atoms to penetrate between the metal atoms, creating the strain = dislocations).

However, as we know, hard things can become brittle. If the hardened object is subjected to a greater force, the dislocations that were stuck in place are forced to move, and at some point the bonds between the atoms break. This is the beginning of fracture. In other words, the object becomes hard for a certain amount of force.
What happens when you get small?
The weakest links (dislocations) in the bonds between the atoms and molecules that make up a substance are present in the substance at a certain rate. This means that the larger the material, the more weak points there are. This means that larger materials are more likely to crack and shatter when subjected to a force.
However, as the size of the material decreases, the number of atoms and molecules that make up the material decreases. Furthermore, the probability of the existence of weak parts is the same as for larger objects. Therefore, the number of weak parts decreases.
So how much does it decrease? For simplicity's sake, let's consider a square material with no thickness. For a material with very few dislocations, the number of dislocations in a square centimetre is estimated to be around 106 to 108 (depending on the literature, the number may vary by one or two orders of magnitude). So what happens to the number of dislocations as the length of the edge is reduced and the square becomes smaller? In the graph below, the vertical axis shows the number of dislocations in the square, and the horizontal axis shows the length of the side of the square. The eight green bars indicate the upper and lower limits of the number of dislocations, and the light blue horizontal dashed line indicates where the number of dislocations is 1. If the number of dislocations is less than or equal to the decimal point, it means the probability of the dislocation being in that square. For example, 1/100 means that if there are 100 squares, there is one dislocation in each of them.
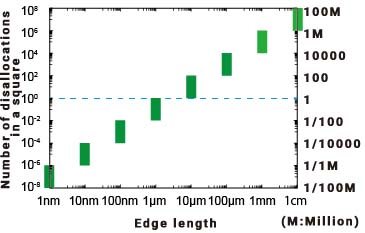
However, as the size of the material decreases, the number of atoms and molecules that make up the material decreases. (You will also notice that the spacing between the smaller scales changes compared to the regular scale. (This is due to the nature of logarithms. This is due to the nature of logarithms. You can read more about logarithms here).
As can be seen from the graph, the number of dislocations decreases by two orders of magnitude for every tenth of a side length (the area is proportional to the square of the length), which means that the number of weak points decreases rapidly with decreasing particle size.
Furthermore, below the submicron level, the number of weak points is almost non-existent (if there are many squares, only a few of them have dislocations).
