- Industries & Machines Industries & Machines
- IIoT IIoT
- Service-Toll Processing Service-Toll Processing
- Material Material
- News News
- IR Information IR Information
-
Sustainability
Sustainability
Sustainability
- Introduction
- Hosokawa Micron Group "Basic Human Rights Policy"
- Hosokawa Micron Group "Basic Policy on the Environment"
- Sustaibality Policy - Mission Statement
- Editorial Policy
- Integrated Report
- Materiality & Strategy
- Technological contribution to a sustainable global environment
- Contributions towards a safer, more secure and prosperous society
- Sophistication of governance that supports business
- ESG Data Collection
- Sustainable Business Management ~ Finance
- Infromation Disclosure Based on TCFD Recommendations
- Jobs and Careers Jobs and Careers
-
About Us
About Us
About Us
- Greetings (Company Introduction)
- Hosokawa Micron Group "Basic Human Rights Policy"
- Hosokawa Micron Group "Basic Policy on the Environment"
- Management Philosophy
- Corporate Overview
- Executive Officers
- Corporate Profile
- Business Areas and Strengths
- Corporate History
- Hosokawa Micron Group
- Domestic Facilities
- Overseas Subsidiaries (Asia)
- Overseas Subsidiaries (Europe)
- Overseas Subsidiaries (America)
- Asian Agents
- Powder Technology Research Institute
- Annual Publication "Micromeritics"
- Industrial Property Rights
- Journals and Books
- Technical Information
- Compliance Charter
- Privacy Policy
- Cookie Policy
- Quality Principle

Industries & Machines
- TOP
- About Us
- Technical Information
- Details of Millling technology
- Relationship between particle size and adhesion
- Greetings (Company Introduction)
- Hosokawa Micron Group "Basic Human Rights Policy"
- Hosokawa Micron Group "Basic Policy on the Environment"
- Management Philosophy
- Corporate Overview
- Executive Officers
- Corporate Profile
- Business Areas and Strengths
- Corporate History
- Hosokawa Micron Group
- Domestic Facilities
- Overseas Subsidiaries (Asia)
- Overseas Subsidiaries (Europe)
- Overseas Subsidiaries (America)
- Asian Agents
- Powder Technology Research Institute
- Annual Publication "Micromeritics"
- Industrial Property Rights
- Journals and Books
-
Technical Information
- Summury of Powder Technology
- Details of Millling technology
- Details of Classification Technology
- Details of Mixing/Blending Technology
- Details of Particle design Technology
- Details of Agglomeration Technology
- Details of Drying Technology
- Details of Dedusting Technology
- Details of Measuring Technology
- Compliance Charter
- Privacy Policy
- Cookie Policy
- Quality Principle
Relationship between particle size and adhesion
Why does fineness make it easier to stick?
As solids decrease in size, they tend to become more adhesive.
This leads to some seemingly strange phenomena.
To illustrate this, let's start with a phenomenon that may seem obvious.
Consider, for example, carrying a bucket of stones or sand.
You have two buckets of the same shape and size. Suppose each bucket is filled with stones or sand in the following two conditions
(1) A stone of 5 cm
(2) 1 mm grains of sand
For simplicity's sake, we assume the following conditions
- Fill the bucket from a certain height and at a certain speed : After filling the bucket to the point where it spills over the edge, scrape it off so that it is as level as possible.
- Material: Both the pebbles and the sand grains should be made of the same material (true density and chemical composition).
- Surface condition: the effects of moisture and static electricity should be negligible.
- Size: No stone or grain of sand is assumed to be larger than 5 cm or 1 mm.
- Shape: the stone should be in a state similar to that found in the middle of a river (close to a sphere but irregular in shape).
The two buckets are (2) heavier than the other.
The reason is simple, but let's explain it in passing.
Each stone is heavier than the other, but because of the irregular shape of the pebbles, there are more gaps between them.
Sand grains, on the other hand, are lighter because they are smaller, but the gaps between them are smaller, so they are heavier.
Now, in order to carry a lot, we need to fill the gaps.
To do this, we can either make the particles flat, or stack them neatly in a rectangular shape.
The former is a problem because few materials can be flattened (metals, mica, etc.) and the latter is almost impossible to produce industrially.
The latter is almost impossible to produce industrially. The irregular shape also makes it difficult to fill because the corners get caught.
The most common method is to make the particles spherical, so that they do not get caught.
However, even with spherical particles, if there is only one particle size, the amount that can be filled tightly is fixed, with a minimum gap of approximately 26%.
For example, a 500 ml PET bottle filled with spherical particles of uniform size, such as glass beads, will only hold 370 ml (the rest being air).
This number was predicted by Johannes Kepler, who proposed the laws of motion of celestial bodies in the solar system (Kepler's laws), which you learn about in high school physics, and is called the Kepler prediction.
It took him roughly 400 years to fully prove it (99% in 1998, fully proven in 2014), and he did so by using computers to study every individual case.
A quiet story.
Now, you might think that if you carry it, you don't need to pack it in so much, so let's list some of the most pressing filling problems related to our daily lives.
For example, in a lithium-ion battery, when the battery is charged, lithium ions are absorbed by the particles of graphite and other materials used as the anode. This means that, in general, the more anode material there is, the more lithium ions can be stored, and therefore the longer the battery can be used (the more capacity it has).
The problem is that the size of the battery is usually fixed (e.g. A3 size) or that the smaller the battery, the easier it is to use (more freedom in the layout of the interior of an electric car).
For this reason, packing as many particles as possible into a fixed deposit is directly related to the longevity of the battery on a single charge.
(Of course, there is more to it than this, both in terms of the material itself and in terms of the particles.
For more information on the latter, please refer to the page on graphite as anode material for rechargeable batteries)
This is just an introduction, but let's get down to business.
If we take the above idea to its logical conclusion, we can say that in order to fill the gaps between large powders, we need to prepare powders that are large enough to fill the gaps, and then fill the smaller gaps that are created by filling the gaps with smaller powders, and so on. The reality is that the maximum number of particles is not enough.
In reality, the maximum size of a particle is often fixed (its size in a container, its thickness in a film) and the difference between the particles to be filled is not uncommon to be a few micrometres or even less.
The curious thing about powders is that when they are reduced in size to less than 10µm, even spherical particles are less likely to clog. The reason for this is that the adhesive forces acting on the particles prevent them from filling.
You might think that the smaller the particle, the greater the adhesive force, but this is not the case.
There are many forces acting on particles. In particular, when there are more than two particles, there are also forces acting between the particles.
There are four known forces that are involved in the adhesion of particles.
- Gravitational force
- Electrostatic force
- Liquid bridge forces (and sometimes solid cross-linking forces)
- van der Waals force
For example, consider the case of a particle sticking to a vertical wall.
The balance of these four forces determines whether the particle will stick to the wall, and how much force will be needed to pull it apart.
We will look at them one by one. The calculation of the specific magnitudes will follow.
Gravity force
It is the force that pulls the particle away from the wall (tries to make it fall) and therefore prevents it from sticking.
This force, Fg, is proportional to the mass of the particle. (The subscript means what it comes from or what it acts on. In this case it means gravitational force). The force Fg can be calculated from Newton's laws of motion using the following formula
Fg=mg = (π/6・d3ρ)
where, m is the mass of the particle (kg), g is the gravitational acceleration [about 9.8 (m/s2)], π is the circumference (-), ρ is the true density of the particle (kg/m3) and d is the particle size [diameter] (m).
Electrostatic force
If the wall and the particle are charged with the same sign, they will repel each other, which is a force that moves the particle away from the wall.
It is known that when a charged object (not just a particle) approaches a wall, even an uncharged wall becomes charged with the opposite sign. This is called dielectric polarisation.
(For this reason, a material that does not conduct electricity, i.e. an insulator, is also called a dielectric. (For this reason, a material that does not conduct electricity is also called a dielectric.)
Even if the wall is a conductor, the same phenomenon will occur if it is insulated.
As a result, particles and walls are attracted to each other.
The electrostatic force Fe in this case is approximately inversely proportional to the square of the ratio of the particle size to the particle-wall distance, although the formula is different depending on whether the particle is a conductor or a dielectric.
Fe∝1/(d/r)2
where, d is the distance (m) and r is the particle size (m). (The subscript e in F stands for electrostatic.) Thus, when the particle-wall distance remains the same, the electrostatic force decreases very quickly (by a factor of 4 for a particle of half the size, and by a factor of 100 for a particle of 1/10 the electrostatic force) as the particle size decreases.
Liquid bridge forces
It is the force between a particle and another object (in this case a wall) that causes the liquid to form a bridge, attracting the objects at either end of the bridge.
Under normal atmospheric conditions, there is always a small amount of water on the surface of the particles, so this force is present even in dry powders.
A number of models have been proposed to describe the liquid bridge force Fl (where l stands for liquid bridge).
In the simplest model, the approximate value of the liquid bridge force Fl is proportional to 1/4 power of the volume of the liquid bridge, 1/4 power of the radius of the particle and proportional to the surface tension.
The volume of the liquid cross-link depends on the wettability of the particle in the liquid.
van der Waals force
Even in electrically neutral substances, there is a force acting between electric dipoles which is instantaneously created on the surface of the substance by the influence of other objects or electric fields. This force was originally conceived as a force acting between molecules.
The van der Waals force between a sphere and a plane, Fw (where, w is Waals' w, and the form of the equation changes between sphere and sphere and plane and plane), is inversely proportional to the square of the distance and proportional to the size (diameter) of the particle.
However, the constant of proportionality is very small, about 10 to the 20th power (10-20; 0.0 -- omitted -- 01),, and there are 19 zeroes after the decimal point. In this case, the unit of the constant of proportionality is the joule, the unit of energy). So you can see that it is a very small force.
Now, in general, as the particle size decreases, all of the above forces decrease.
Therefore, it is not correct to say that the adhesion force increases as the particle size decreases.
The graph below shows how these four forces decrease with particle size.
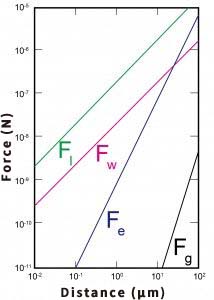
The unit on the vertical axis of the graph is written as N, which is read as Newton. (Named after Newton, famous for the law of universal gravitation.)
Now, let's review.
- Force to pull apart: Fg, Fe when the particle and the wall are of the same sign
- Force to adhere: Fe, Fl and Fw for the case of different signs.
The graph shows that the gradients of Fg and Fe are steeper than those of Fl and Fw. This means that as the particle size decreases, the difference between these forces becomes increasingly larger. Therefore, the force of attachment ≥ the force of detachment, or the force of attachment ≫ the force of detachment
Therefore, the smaller the particle size, the more adhesive it will be.
Supplement: Forces and equations of motion
1N is defined as the force that causes an object with a mass of 1kg to accelerate by 1 metre per second per second (m/s2).
Acceleration is a bit complicated, but Newton's equation of motion says that it is.
F=ma, or force, is mass multiplied by acceleration.
The equation has a cleaner form without the proportionality constant, but that is because the units have been adjusted, as noted above, so that the proportionality constant is 1.
Incidentally, this formula does not appear in Philosophia Naturalis Principia Mathematica (abbreviated to Principia), Newton's work on mechanics. It was formulated in this form by Leonhard Euler. Euler has done a great deal of work in the field of mathematical physics, including Euler's formula eiθ=cosθ+ i sinθ and the most beautiful equality in the history of mathematics, eiπ=-1, derived from it. Here, e is the base of the natural logarithm and i is the imaginary unit.
